At Quinten MD, with the rise of new advanced technologies and the development of our analytic capabilities, we analyze Real-world data (RWD), generate real-world evidence (RWE) and apply it to medical devices for regulatory compliance.
Real-World Data (RWD) and Real-World Evidence (RWE)
RWD and RWE play an increasing role in healthcare decisions.
The global healthcare community is increasingly using patient data from real-world settings to derive actionable insights that are not typically captured in traditional randomized controlled trials (RCTs).
Real-World Data (RWD)
RWD is feined by the U.S. Food and Drug Administration (FDA) as the data relating to patient health statut and/or the delivery of healthcare routinely collected from a variety of sources.
RWD can come from a number of sources, for example:
• Electronic Health Records (EHRs)
• Claims and billing activites
• Product and disease registries
• Patient-generated data including in home-use settings
• Data from other sources than can inform on health status, such as mobile devices
Real-World Evidence (RWE)
According to FDA, RWE is the clinical evidence regarding the usage of potential benefits, or risks of a medical product derived from analysis of RWD.
RWE is extracted form RWD through the application of research methods, including studies of a wide variety of deisgns, referred to as Real-World Evidence studies.
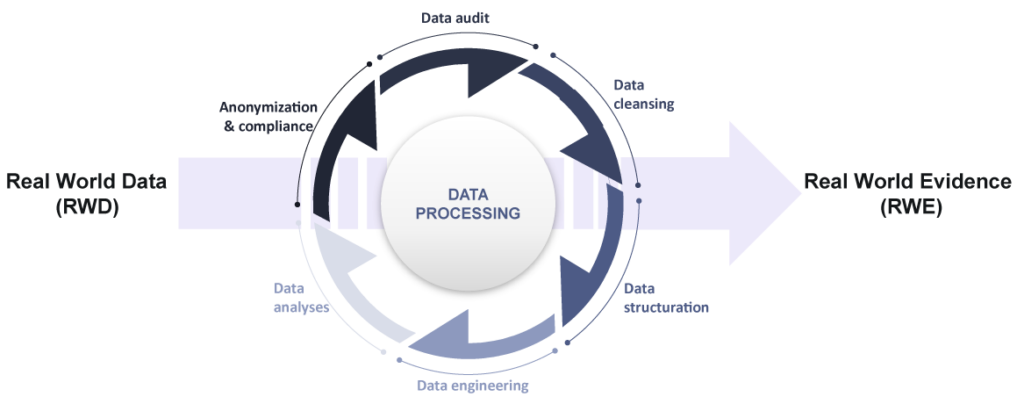
RWE derives from the transformation ant the analysis of RWD
Electronic Medical Records (EMR) and hospital data used in our studies
Our studies are conducted on RWD originated from Electronic Medical Records (EMR) and hospital systems, collected in French hospitals that are part of our partner hospital network.
Electronic Medical Records (EMRs) are the digital version of a hospital’s medical and treatment history. The anonymous patient data are collected from patient management software used by physicians and healthcare staff to document patient’s clinical records.
Data from EMR are completed by other hospital data sources providing information into what happens and how patients are being treated during hospital visits.
Key information collected (non-exhaustive)
Demographic data
Procedures
Medical history
Diagnosis history
Medical, Paramedical acts
Family history
Prescriptions
Lab results
Hospital movements
Dispensed treatment
Hospital visits
Providers data
Benefits of using RWD et RWE in our studies
The Medical Device Regulation (MDR) requires proactive lifecycle data collection on normal medical device use. RWE generated from our studies meets MDR requirements to prove safety and performance of the device under normal use.
We have chosen to conduct retrospective studies based on the use on RWD because when properly collected, structured, and analyzed, RWD can bring unique and valuable insights into the performance and safety of medical devices.
The data obtained from real-world settings reflects actual routine clinical practice and fills in knowledge gaps that the RCT did not. Using RWD, we collect data related to the use of the device by the population which is really exposed to the device and we can include a high number of patients from multiple clinical sites.
Assessing RWD to support regulatory decision-making for your device
We know that finding clinical data for your device is essential, but it is a long, difficult and daunting process. Quinten MD makes it easy by providing a combined solution of clinically-valuable data, advanced analytics and expertise to meet your MDR compliance needs for your devices. While ensuring patient health data is protected, performance is confirmed, and safety can be strongly entrenched.
➔ Regulatory processes are completed faster, and your devices stay in the market.