Trusted Real World Evidence
for Medical Device compliance
We provide you with clinical data and generate clinical evidence to support your regulatory processes and MDR compliance.
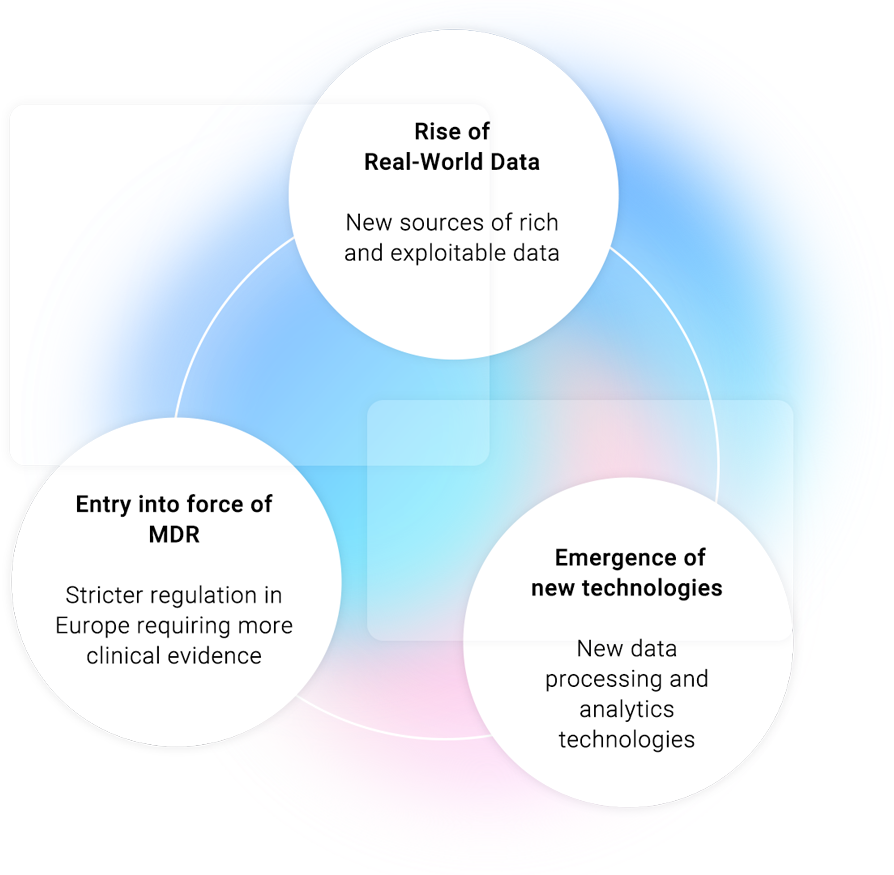
Our innovative model enhances the clinical evaluation of medical devices, reducing costs & delays and minimizing risk in order to strengthen the compliance with the Medical Device Regulation (MDR) requirements and deliver relevant data to Notified Bodies.
OUR OFFER
Retrospective clinical studies for high-risk medical devices based on Real-World Data (RWD)
Our services are fully in line with the context of the new regulations.
We use our advanced analytics and AI capabilities, as well as our proven expertise in Real-World Evidence (RWE) generation and conduct retrospective clinical studies for high-risk medical devices based on RWD.
Find out more about our services
Want to know more about Real-World Data and Real-World Evidence?
Please visit our page describing the data we use in our studies
YOUR NEEDS
Harness the potential of Real-World Data to confirm safety and performance
- Transfer existing medical device certificates into MDR
- Collect clinical evidence for the Post-Market Clinical Follow-up
- Implement changes to product, intended use or indications for use
- Address unanswered questions of long-term safety and performance
- Renew the CE mark to keep your device on the market
- Collect more evidence and more data on your devices
- Handle specific requests from your Notified Body
- Establish an ongoing process for collecting clinical evidence over time for your device
Let’s explore your needs together
BENEFITS OF WORKING WITH US
Accelerating regulatory success with RWE
• Less than one year from contracting to results delivery
• Secured and defined trial design
• -30% cost in average (compared to clinical investigation)
• Transparent and flat fees from-end-to-end
• High Quality and MDR compliant evidence
• Real-World Evidence (RWE) supported by regulatory authorities
Want to take advantage of this benefit?
KEY FIGURES
13+
years in Healthcare and Medical Devices
5
years in Real World Evidence Generatio
360°
in-house dedicated expertise and academical background
OUR TEAM
Meet Quinten MD team
We are a dynamic and experienced consultants team of Clinical Project Managers, Biostatisticians, Data Analysts, Data Scientists and Clinical Research Assistants who understand the challenges of the medical device industry and want to put their expertise at the service of the manufacturers.




Need more information regarding our solutions, our expertise or our company?
Fill out our contact form, we will answer you within 48 hours